Context
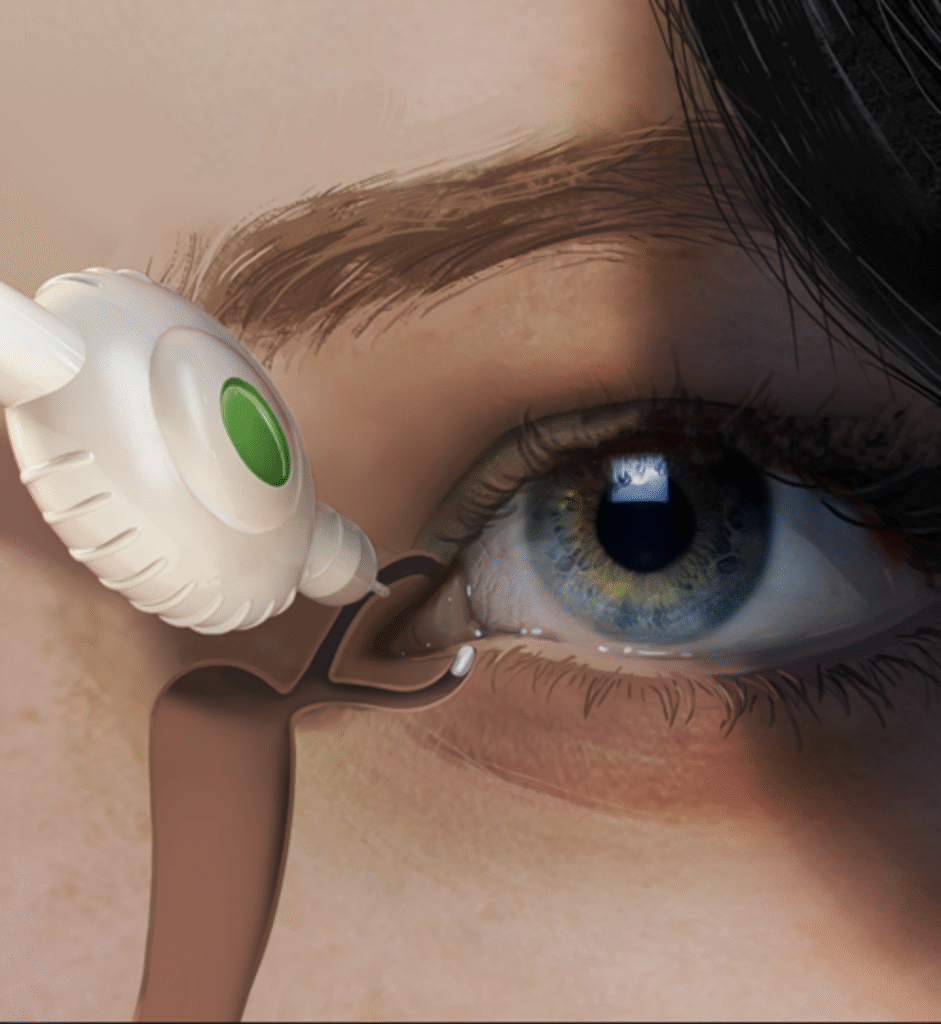
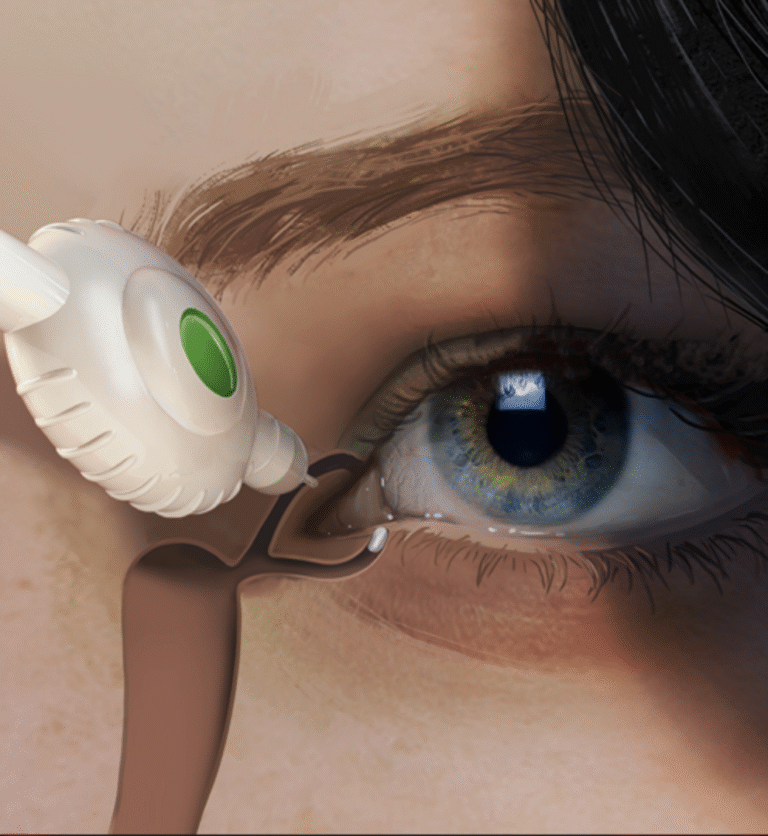
A Series A/B ophthalmology biotech partnered with iO Lifescience to develop the first prototype of a precision intravitreal delivery system for high-viscosity biologics. The aim: create a surgeon-friendly, scalable solution capable of delivering micro-volume doses with accuracy and consistency, ready for pre-clinical and early clinical evaluations.
Challenges
The program required combining micro-mechanical precision with clinical usability:
- Viscosity & dose control: Deliver tens of microliters through 30–33G needles with controlled force and no shear degradation.
- Human factors: Enable one-handed use with tactile cues and aspiration safeguards for retina specialists.
- Container & materials: Use low-silicone, tungsten-free components with validated container-closure integrity.
- Scalability: Co-develop with tier-1 suppliers for moldable, sterilizable, and assembly-ready parts.
- Speed: Produce evidence-ready prototypes for pre-clinical and workflow validation within weeks.
iO Lifescience delivered systems design, container selection, bench testing, human-factors studies, packaging rationale, verification strategy, and pilot manufacturing plans.
Outcomes
- Concept-to-alpha in 10 weeks, beta units in 14 weeks for pre-clinical testing.
- Precision verified: Target micro-volumes delivered through 30–33G needles within 7 seconds, ≤10 N plunger force.
- ≥95% task success; design locked for summative testing.
- Manufacturing readiness: Geometry and DFM frozen with high-volume suppliers.
- Regulatory foundation: RMF, usability risk analysis, and verification matrix compiled for pre-clinical approval path.