Context
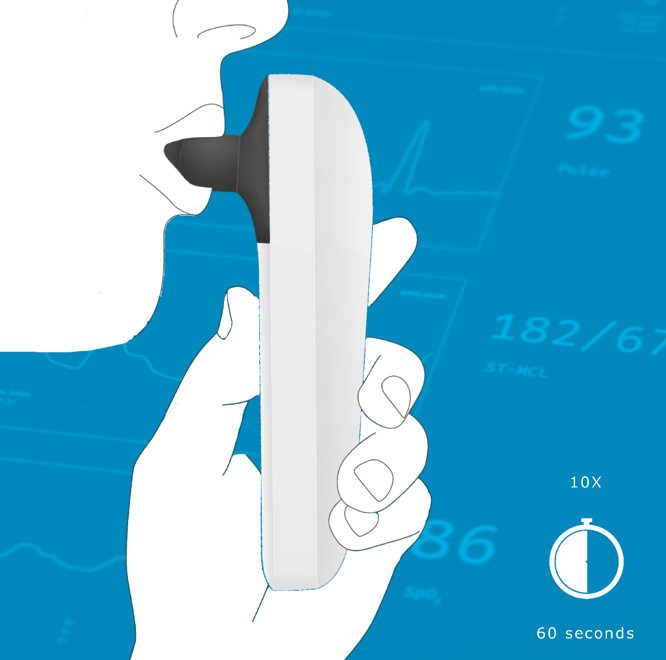
A pre-seed neuro-health startup partnered with iO Lifescience to transform an early concept into functional prototypes of a non-invasive, head-worn neuro-assessment device with a companion mobile app. The goal: enable fast, repeatable neurological testing outside specialized clinics, supporting the evolution of connected digital care pathways.
Challenges
The project required rapid design execution, reliable biosignal acquisition, and human-centered usability:
- Rapid prototyping: Translate clinical sketches into EVT prototypes with integrated sensors, firmware, and mobile app architecture under ISO 13485 controls.
- Human factors: Fit across head sizes and hair types, intuitive don/doff, and safe skin-contact materials (IEC 62366-1).
- Signal reliability: Achieve stable readings during movement with optimized shielding, filtering, and artifact rejection.
- Regulatory groundwork: Develop ISO 14971 risk file, URRA, cybersecurity plan, and pre-submission roadmap.
- Scalable manufacturing: DFM/DFA for injection-molded housings, flex-rigid PCBs, elastomer interfaces, and supplier qualification for pilot tooling.
iO Lifescience provided systems design, firmware, app stub, usability studies, risk management, verification, and supplier sourcing.
Outcomes
- Human factors: ≥90% success across key tasks; setup time <2 minutes.
- Manufacturing readiness: Locked tool-ready geometry, BOM, and COGS with qualified suppliers.
- Regulatory preparedness: Delivered risk files, verification matrix, and cybersecurity assessment for pre-submission.
- Investor traction: Prototype and usability data supported follow-on funding and early clinical-site engagement.