A methodology for success
Compliant human-centric innovation
Start with the users
Understand the problem you are solving before designing the answer. Ask the right questions, integrate user empathy and clearly define requirements.
Assemble the experts
The right team with the right expertise is key to executing on your vision. We will assemble the best minds in medical device design, engineering and compliance.
De-risk while adding value
By plotting deliverables, verifying along the way and always keeping compliance in mind, risk quickly declines and value significantly increases throughout the entire process.
Rapid de-risk driven ROI
Through a capital efficient model and ongoing verification we
de-risk execution to quickly drive value and innovation.
01 feasibility
immediate de-risking and value with user insights
02 ideation
deliver proof-of-concept device prototypes
03 development
accelerate concept-to-trials through risk-managed models
04 compliance
ISO 13485 documentation for faster time-to-market
Rapid de-risking leads to ROI
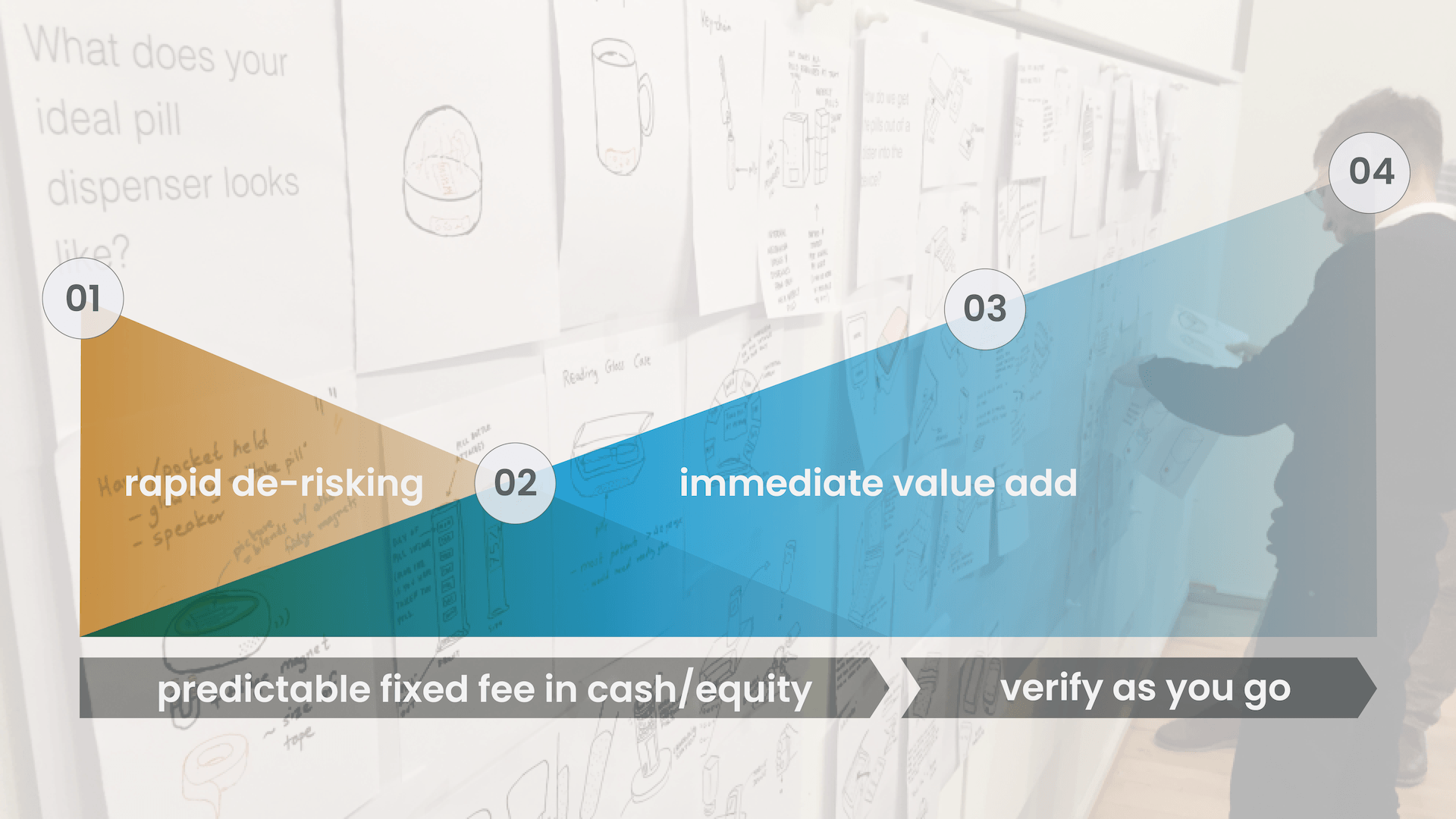
01 proposal & feasibility
immediate de-risking and value with user insights
02 design innovation
deliver proof-of-concept device prototypes
03 development execution
accelerate concept-to-trials through risk-managed models
04 compliance assurance
ISO 13485 documentation for faster time-to-market
Through a fixed fee model and ongoing verification we rapidly de-risk execution to quickly drive value and innovation.
Purpose led innovation
From the start of the development process it is important to search for meaning and synthesize user insights in order to explore the possibilities that lead to design innovation.
cost predictability • accelerated time-to-market • devices users love
